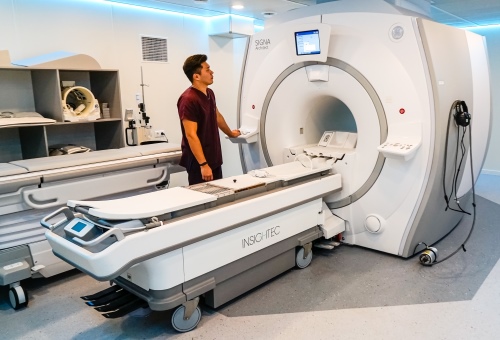
Focused ultrasound treatment center. Multidisciplinary center of oncology and surgery (Ust-Kamenogorsk)


For the first time in Kazakhstan. Focused Ultrasound Ablation Guided by Magnetic Resonance Imaging MRgFUS (FUS-MRI)
Our company “Mediсal Innovations & Technologies” has implemented an innovative project to equip the operating unit on the basis of the Public Utility Enterprise at the Right of Economic Use East Kazakhstan Region (EKR) “Multidisciplinary Center of Oncology and Surgery” Health Department of EKR in Ust-Kamenogorsk. The key elements of the project were the ExAblate system for non-invasive ablation by focused ultrasound from INSIGHTEC and the 3.0T SIGNA Architect magnetic resonance imager from GE Healthcare.
INSIGHTEC equipment is widely known in the world for its amazing results, leading clinics can no longer imagine their work without it. Now this system is also available in Kazakhstan.
About the technology
Ablation by Focused Ultrasound Controlled by Magnetic Resonance Tomography (MRgFUS) is a non-invasive surgical procedure in which Focused Ultrasound (FUS) remotely coagulates clearly defined areas of specific tissues within the body under the control of magnetic resonance imaging (MRI).
High technology MRgFUS (FUS-MRI) ablation allows non-invasive removal of tumors, nerve endings, which are sensory sources of pain, as well as a point effect on pathways in the brain.
MRgFUS (FUS-MRI) is a combination of high-intensity focused ultrasound, which provides coagulation of selected points inside the body under the control of magnetic resonance imaging, which provides visualization of anatomical structures, guidance of ultrasound and control of heating in real time.
Operations using MRgFUS technology (FUS-MRI) are performed outpatiently and do not require anesthesia. The duration of MRgFUS procedure (FUS-MRI) depends on the volume of tumor nodes and their quantity, on average, is 3 hours.
Application in oncology:
- Prostate Cancer Focal Therapy (CE)
- Palliative care for bone metastases and pain syndrome (FDA, CE)
- Treatment of uterine myoma and adenomyosis (FDA, CE)
Previously, this procedure was available only abroad, now that “Mediсal Innovations & Technologies” has implemented this medical solution in Ust-Kamenogorsk, this procedure has become easily and quickly available to all residents of Kazakhstan.